No one ever celebrates mucus. Doesn’t matter if it’s clear and watery (or goopy and green), most people blow their noses and throw out the tissue without a second thought. But let’s take a closer look…

Mucus is called phlegm when it’s in the respiratory tract, but once it’s coughed out, it becomes sputum. Disgusting and oddly fascinating at the same time. [Image from a completely unabashed Wikipedia user.]
Sure, mucus can be gross (after all, it’s filled with all the dust, pollutants, and bacteria you don’t want in your lungs), but it’s also the product of the millions of hair-like protrusions that beat in synchrony, lining our airways like so many tiny street-sweepers.
So many cilia, beating and sweeping in unison.
The city of San Diego might boast about its twenty motorized sweepers, but each individual respiratory epithelial cell has approximately 200 cilia!1 These cilia are tireless, beating 8x per second2 and constantly pushing out mucus—like a modern-day, mini Sisyphus (except successful).
Occasionally though, cilia do fail. A rare autosomal recessive disease, Primary Ciliary Dyskinesia (PCD) is characterized by defects in cilia movement, whether the cilia are immotile, sweep asynchronously, or even beat too fast. Without functional cilia, mucus can’t clear (causing chronic respiratory problems), sperm can’t swim (causing infertility), and there are even defects in basic left-right body organization (situs inversus). And if you’re wondering why PCD sounds so familiar, this isn’t the first time that it’s shown up on our Gene-of-the-Week blog.
Given the complexity of cilia structure (there’s at least 250 proteins associated with it!),3 PCD can arise from many different gene mutations. There have already been PCD-causing mutations reported in more than twenty different genes,4 including the current Gene-of-the-Week, DNAH11.
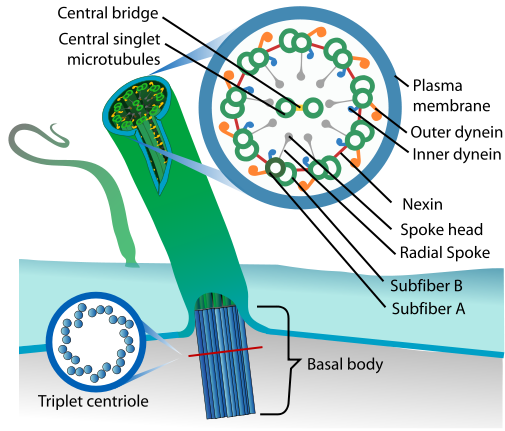
Read Kerin’s #GeneOTW on CCDC40 to learn more about cilia structure, and another mutation that causes PCD. [Image from Wikipedia.]
DNAH11 (dynein axonemal heavy chain 11) is an outer dynein arm protein, which acts as a molecular motor with ATPase activity; simply put, it’s responsible for the cilia’s stereotyped beating pattern. Unlike most causes of PCD (cough, CCDC40), mutations in DNAH11 don’t actually affect cilia structure, just their movement. Depending on the location of the mutation, DNAH11-mutant cilia can have reduced waveform amplitude, hyperkinetic beating, or can even be entirely immotile.56 This so-called “PCD with normal ultrastructure” often goes undiagnosed, since you need to analyze the cilia beat pattern with high-speed video microscopy (a difficult technique with limited availability). Still, now some of these cases can be confirmed by sequencing for DNAH11 mutation. There’s no cure for faulty cilia, but at least a definite PCD diagnosis will help inform treatment options that can offset PCD’s respiratory problems and slow progressive damage to the lungs.
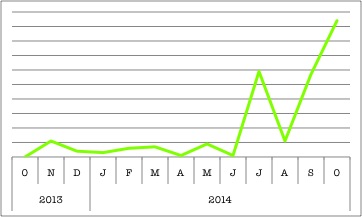
Clearly, PCD is a hot topic and cilia are pretty important. Why else would there be multiple ciliary genes in BioGPS’s top search queries? These organelles might be tiny, but they definitely have a big impact. (This chart shows DNAH11’s rise, but check out CCDC40 here.)
So now, when flu season comes around (and you’re buried in a mountain of used tissues or hacking out your lungs), take comfort in knowing that all the gunk in your nose and chest has a purpose. And if you’re not the squeamish type, watch this endoscopic surgery video and marvel at some real-time mucociliary clearance! Regardless, just take time to thank your mucus, applaud your cilia, and truly appreciate the fine-tuned biological machinery that keeps our bodies running.
REFERENCES:
- Wanner A, Salathé M, O’Riordan TG. (1996) Mucociliary clearance in the airways. Am J Respir Crit Care Med 154(6 Pt 1):1868-902. [↩]
- Boon M, Smits A, Cuppens H, Jaspers M, Proesmans M, Dupont LJ, Vermeulen FL, Van Daele S, Malfroot A, Godding V, Jorissen M, De Boeck K. (2014) Primary ciliary dyskinesia: critical evaluation of clinical symptoms and diagnosis in patients with normal and abnormal ultrastructure. Orphanet J Rare Dis 9:11. [↩]
- Lucas JS, Adam EC, Goggin PM, Jackson CL, Powles-Glover N, Patel SH, Humphreys J, Fray MD, Falconnet E, Blouin JL, Cheeseman MT, Bartoloni L, Norris DP, Lackie PM. (2012) Static respiratory cilia associated with mutations in Dnahc11/DNAH11: a mouse model of PCD. Hum Mutat 33(3):495-503. [↩]
- Boon M, Smits A, Cuppens H, Jaspers M, Proesmans M, Dupont LJ, Vermeulen FL, Van Daele S, Malfroot A, Godding V, Jorissen M, De Boeck K. (2014) Primary ciliary dyskinesia: critical evaluation of clinical symptoms and diagnosis in patients with normal and abnormal ultrastructure. Orphanet J Rare Dis 9:11. [↩]
- Lucas JS, Adam EC, Goggin PM, Jackson CL, Powles-Glover N, Patel SH, Humphreys J, Fray MD, Falconnet E, Blouin JL, Cheeseman MT, Bartoloni L, Norris DP, Lackie PM. (2012) Static respiratory cilia associated with mutations in Dnahc11/DNAH11: a mouse model of PCD. Hum Mutat 33(3):495-503. [↩]
- Pifferi M, Michelucci A, Conidi ME, Cangiotti AM, Simi P, Macchia P, Boner AL. (2010) New DNAH11 mutations in primary ciliary dyskinesia with normal axonemal ultrastructure. Eur Respir J 35(6):1413-6. [↩]
