Most people have at least 10 of these on their bodies. Some even have up to 40.1 Occasionally, just one is enough to make you famous. What are we talking about here? Moles, of course! As a common example of a benign tumor, moles happen to nearly everyone, and are (generally) not a cause for worry. However, not all benign tumors are as harmless…

Cindy Crawford is famous for her trademark mole. Moles may technically be benign tumors, but they’re also called beauty marks. Indeed, they’re coveted so much that people sometimes get “Monroe piercings” to simulate that glamorous movie star look! Too bad not all benign tumors are as desirable… (Wikimedia Commons)
By definition, benign tumors are distinguished from malignant ones by their inability to invade other tissues. Without the capacity for metastasis, a tumor isn’t considered cancerous—but that doesn’t mean it can’t be dangerous. For patients with a rare disorder called Carney Complex, many symptoms arise from the negative effects of “benign” tumors.
As a rare autosomal dominant disorder, Carney Complex has only been identified in 750 patients worldwide since 1985.2 Typical clinical features include spotty skin pigmentation, (benign) endocrine tumors, and (benign) cardiac myxomas. Despite being unable to metastasize, these benign tumors can do plenty of damage on their own. In fact, endocrine tumors in the adrenal glands can produce excess hormones, leading to Cushing’s Syndrome. However, cardiac myxomas are far more detrimental; indeed, most patients with Carney Complex have a significantly decreased life-span, due largely to heart-related fatalities.3 If left untreated, cardiac myxomas can break apart, enter the circulation, and cause embolic stroke by blocking an artery; if the myxoma grows large enough, it can even obstruct blood flow in the heart and cause sudden death.
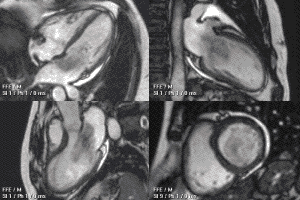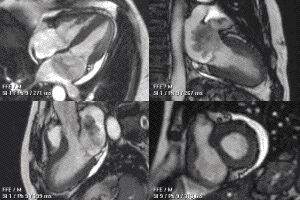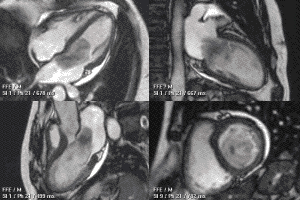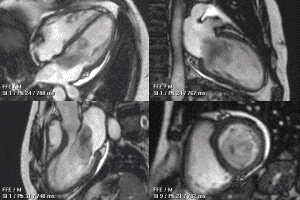
This MRI of the heart shows a large myxoma as the dark mass that plunges back and forth between the atrium and ventricle. (Wikimedia Commons)
Even though tumorigenesis is a primary feature of Carney Complex, the mechanisms leading to this pathology remain unclear. Because most cases of Carney Complex are caused by mutations in the PRKAR1A gene (Protein Kinase A, regulatory subunit, type 1a), it’s assumed to be a tumor suppressor gene. However, the data is (unsurprisingly) a bit more perplexing. Loss of PRKAR1A protein reliably leads to an increase in Protein Kinase A (PKA) activity, but depending on the context, PKA activity has been shown to both increase cell proliferation and apoptosis.4
3D structure of the PRKAR1A protein. (Wikimedia Commons)
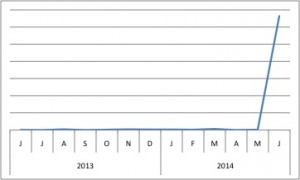
PRKAR1A has skyrocketed in popularity (as measured by BioGPS), likely due to the recent publication that disputes current views of PRKAR1A’s role in tumorigenesis.
Adding to the controversy, a recent publication demonstrates that mutation in PRKAR1A alone actually induces apoptosis—exactly opposite and contradictory to the idea that disruption of PRKAR1A (as seen in Carney Complex) induces tumors.5 It’s only in conjunction with additional mutations (to oppose the pro-apoptotic effects), that PRKAR1A mutants recapitulate the hallmark Carney Complex characteristic of tumorigenesis.6 Together, this suggests that the etiology of Carney Complex is more complicated than expected, involving not only mutations in PRKAR1A, but perhaps also mutations or epigenetic modifications to other genes. And so, just as “benign” tumors aren’t always benign, sometimes “tumor suppressor genes” actually have an opposite role than what we suspect—science may occasionally be unpredictable, but at least it’s always exciting!
References:
- “Common Moles, Dysplastic Nevi, and Risk of Melanoma.” National Cancer Institute. Retrieved 25 July 2014 from http://www.cancer.gov/cancertopics/factsheet/Risk/moles [↩]
- Gangoda L, Doerflinger M, Srivastava R, Narayan N, Edgington LE, Orian J, Hawkins C, O’Reilly LA, Gu H, Bogyo M, Ekert P, Strasser A,Puthalakath H. (2014) Loss of Prkar1a leads to Bcl-2 family protein induction and cachexia in mice. Cell Death Differ. doi: 10.1038/cdd.2014.98. [Epub ahead of print] [↩]
- Gangoda L, Doerflinger M, Srivastava R, Narayan N, Edgington LE, Orian J, Hawkins C, O’Reilly LA, Gu H, Bogyo M, Ekert P, Strasser A,Puthalakath H. (2014) Loss of Prkar1a leads to Bcl-2 family protein induction and cachexia in mice. Cell Death Differ. doi: 10.1038/cdd.2014.98. [Epub ahead of print] [↩]
- Gangoda L, Doerflinger M, Srivastava R, Narayan N, Edgington LE, Orian J, Hawkins C, O’Reilly LA, Gu H, Bogyo M, Ekert P, Strasser A,Puthalakath H. (2014) Loss of Prkar1a leads to Bcl-2 family protein induction and cachexia in mice. Cell Death Differ. doi: 10.1038/cdd.2014.98. [Epub ahead of print] [↩]
- Gangoda L, Doerflinger M, Srivastava R, Narayan N, Edgington LE, Orian J, Hawkins C, O’Reilly LA, Gu H, Bogyo M, Ekert P, Strasser A,Puthalakath H. (2014) Loss of Prkar1a leads to Bcl-2 family protein induction and cachexia in mice. Cell Death Differ. doi: 10.1038/cdd.2014.98. [Epub ahead of print] [↩]
- Gangoda L, Doerflinger M, Srivastava R, Narayan N, Edgington LE, Orian J, Hawkins C, O’Reilly LA, Gu H, Bogyo M, Ekert P, Strasser A,Puthalakath H. (2014) Loss of Prkar1a leads to Bcl-2 family protein induction and cachexia in mice. Cell Death Differ. doi: 10.1038/cdd.2014.98. [Epub ahead of print] [↩]
