Slightly unsavory (and certainly salacious), the subject of semen is seldom suitable for social settings. But why are we so shy about semen? Scientifically speaking—it’s a pretty spectacular substance.
Besides containing sperm, semen is also comprised of various goodies secreted from the seminal vesicle glands, including proteins, mucus, and even the fructose that supplies energy to the sperm! One especially important component is Semenogelin I (SEMG1), the predominant protein found in semen.1
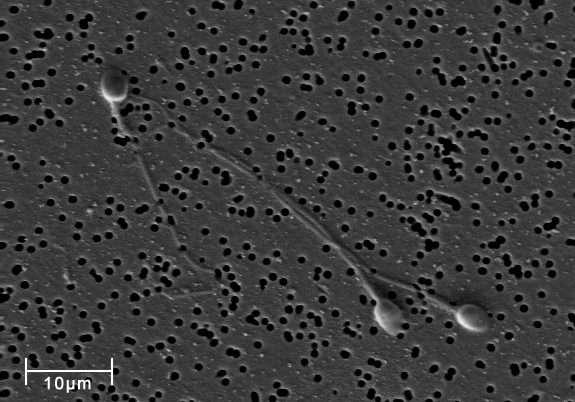
This image just shows three sperm cells, but for each ejaculation, there are over 100 million sperm cells! In addition to sperm (and thus half the DNA required to make new life), semen also contains a plethora of interesting and essential proteins, like SEMG1 (and even lactoferrin, another GeneOTW alumnus).
Immediately following ejaculation, SEMG1 aggregates with epididymal protease inhibitor (EPPIN) and fibronectin, causing semen to become temporarily gelatinous.2 This physically immobilizes the sperm, and SEMG1 and EPPIN further inhibit sperm motility by affecting the intracellular calcium and pH levels in sperm.3 However, within 20 minutes, SEMG1 is enzymatically degraded into smaller protein fragments, causing the thick “semen coagulum” to liquefy… thereby freeing the sperm to swim onward towards the egg.4

Why is it evolutionarily advantageous to have semen coagulum? Some say that it prevents sperm “backflow”. Others speculate that species with more promiscuous females have thicker semen coagulum; if another male mates with the female, his sperm will be subsequently blocked by the first male’s coagulum.5 For example, chimpanzees have many sexual partners, and more coagulated semen than us. (Source: lkiwaner)
That’s not the end of SEMG1’s role though; even as it gets broken down, its smaller fragments continue to be active in a variety of processes. For example, some SEMG1 fragments actually have anti-microbial properties, which protect the sperm as they travel through the relatively hostile female reproductive tract.6 Not all SEMG1 fragments are beneficial though; other fragments aggregate into amyloid fibrils that enhance HIV infections.7 In both cases though, the anti-microbial and HIV-enhancing properties are only temporary, since eventually, SEMG1 will be degraded into tinier, non-functional pieces, finally ending its stint as a protein “jack-of-all-trades”.
But Wait, There’s More!
Increasing our understanding of reproductive processes is fascinating and instructive in itself, but studying SEMG1 could also lead to impactful applications—most excitingly, male birth control! Indeed, reversible sterility was successfully induced in macaque monkeys by manipulating EPPIN function!8 Because EPPIN and SEMG1 interactions inhibit sperm motility, EPPIN-SEMG1 binding sites are considered attractive targets for pharmacological intervention.9 Research, of course, is ongoing.
Recent publications suggest that SEMG1 could be used as a biomarker for certain cancers! SEMG1 could also be increasing in popularity, since male contraceptives have been causing a hubbub in the mainstream media lately.
Another promising application is cancer diagnostics. SEMG1 may be most infamous for it’s role in semen, but it’s actually found all over the body. In the kidneys, SEMG1 expression is being pursued as a possible biomarker for renal cell carcinoma.10 Preliminary data demonstrates that even in the same patient, cells from malignant tumors have lower SEMG1 levels than in normal tissue; similarly, patients with SEMG1-negative tumors have a higher risk of cancer recurrence.11 In this case, SEMG1 could be used to predict cancer progression and severity. Confusingly though, SEMG1 might promote prostate cancer and has been detected in other malignancies like lung carcinoma, melanoma, and leukemias.12 SEMG1 might be expressed in many tissues, but it clearly doesn’t have the same function in each.
Far from mundane, SEMG1 is mysterious and fascinating, with diverse functions beyond its role in semen. And yet, despite having been discovered nearly 30 years ago,13 SEMG1 still has only 150 publications to its name. But really, how surprising is that? After all, how many grad students would feel comfortable telling their parents that they’re devoting the next 5+ years of their life to studying semen? Perhaps it’s time to end the stigma, and stop the shame. Share the love about SEMG1, and spread the word: science is pretty awesome… and so is semen.
REFERENCES:
- Lilja H, Abrahamsson PA, Lundwall A. (1989) Semenogelin, the predominant protein in human semen. Primary structure and identification of closely related proteins in the male accessory sex glands and on the spermatozoa. J Biol Chem 264(3):1894-900. [↩]
- Edström AM, Malm J, Frohm B, Martellini JA, Giwercman A, Mörgelin M, Cole AM, Sørensen OE. (2008) The major bactericidal activity of human seminal plasma is zinc-dependent and derived from fragmentation of the semenogelins. J Immunol 181(5):3413-21. [↩]
- O’Rand MG, Widgren EE. (2012) Loss of calcium in human spermatozoa via EPPIN, the semenogelin receptor. Biol Reprod 86(2):55. [↩]
- Edström AM, Malm J, Frohm B, Martellini JA, Giwercman A, Mörgelin M, Cole AM, Sørensen OE. (2008) The major bactericidal activity of human seminal plasma is zinc-dependent and derived from fragmentation of the semenogelins. J Immunol 181(5):3413-21. [↩]
- Dorus S, Evans PD, Wyckoff GJ, Choi SS, Lahn BT. (2004) Rate of molecular evolution of the seminal protein gene SEMG2 correlates with levels of female promiscuity. Nat Genet 36(12):1326-9. [↩]
- Edström AM, Malm J, Frohm B, Martellini JA, Giwercman A, Mörgelin M, Cole AM, Sørensen OE. (2008) The major bactericidal activity of human seminal plasma is zinc-dependent and derived from fragmentation of the semenogelins. J Immunol 181(5):3413-21. [↩]
- Roan NR, Liu H, Usmani SM, Neidleman J, Müller JA, Avila-Herrera A, Gawanbacht A, Zirafi O, Chu S, Dong M, Kumar ST, Smith JF, Pollard KS,Fändrich M, Kirchhoff F, Münch J, Witkowska HE, Greene WC. (2014) Liquefaction of semen generates and later degrades a conserved semenogelin peptide that enhances HIV infection. J Virol 88(13):7221-34. [↩]
- O’Rand MG, Widgren EE, Sivashanmugam P, Richardson RT, Hall SH, French FS, VandeVoort CA, Ramachandra SG, Ramesh V, Jagannadha Rao A. (2004) Science 306(5699):1189-90. [↩]
- Silva EJ, Hamil KG, Richardson RT, O’Rand MG. (2012) Characterization of EPPIN’s semenogelin I binding site: a contraceptive drug target. Biol Reprod 87(3):56. [↩]
- Zhang S, Fang J, Zhang X, Qin C, Su S, Deng Y, Song Z, Zhang Y, Wang H, Yin C, Wang Z. (2014) Seminal plasma protein in renal cell carcinoma: expression of semenogelin I is a predictor for cancer progression and prognosis. Tumour Biol. 2014 Jun 11. [ePub ahead of print]. [↩]
- Zhang S, Fang J, Zhang X, Qin C, Su S, Deng Y, Song Z, Zhang Y, Wang H, Yin C, Wang Z. (2014) Seminal plasma protein in renal cell carcinoma: expression of semenogelin I is a predictor for cancer progression and prognosis. Tumour Biol. 2014 Jun 11. [ePub ahead of print]. [↩]
- Canacci AM, Izumi K, Zheng Y, Gordetsky J, Yao JL, Miyamoto H. (2011) Expression of semenogelins I and II and its prognostic significance in human prostate cancer. Prostate 71(10):1108-14. [↩]
- Lilja H, Oldbring J, Rannevik G, Laurell CB. (1987) Seminal vesicle-secreted proteins and their reactions during gelation and liquefaction of human semen. J Clin Invest 80(2):281-5. [↩]

Trackbacks/Pingbacks